Bio
Korean drugs to make inroads into US market in 2017
[THE INVESTOR] A number of South Korean biopharmaceutical products will make their full-fledged debuts in the US next year, with the aim of taking a share of the world’s largest market for pharmaceutical and health care goods.
Leading the move is Celltrion, the developer of the world’s first biosimilar drug referencing Johnson & Johnson’s Remicade, which treats chronic inflammatory illnesses such as rheumatoid arthritis and Crohn’s disease.
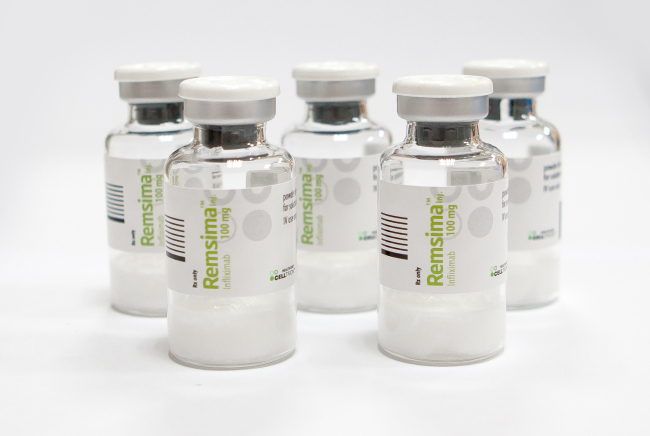 |
Celltrion’s US marketing partner Pfizer officially began sales of Celltrion’s Remicade biosimilar in the US under the trade name Inflectra from late November.
Biosimilars refer to cheaper, near-replicas of biologic drugs which have lost patent protection. Multiple companies around the world have jumped into biosimilars development in line with the approaching patent expiry of some of the world’s top-selling biologics.
Inflectra, also known as Remsima, will be introduced at a 15 percent discounted price from the current wholesaler acquisition cost of Remicade, according to Pfizer.
Celltrion Healthcare, the distribution unit of Celltrion, will have shipped 260 billion won ($216 million) worth of the drug to Pfizer for sales in the US by the end of this year. It expects additional sales to be generated throughout 2017, as more doctors prescribe Inflectra over the more expensive Remicade.
“Remsima (another name for Inflectra) is expected to enjoy exclusive status as the only Remicade biosimilar available in the US for the next two years,” said Daishin Securities analyst Seo Keun-hee.
Moreover, Celltrion plans to file two additional biosimilars, one referencing Herceptin and another Rituxan, for approval by the US Food and Drug Administration early next year.
Upon their approval, the two drugs will be commercialized in the US on behalf of Celltrion by Israel’s Teva Pharmaceutical Industries.
Meanwhile, CSL Behring expects to see next year a boost in sales of Afstyla, a treatment for hemophilia A, co-developed with SK Chemicals.
The next-generation hemophilia drug was commercialized in the US from June, following the US FDA’s official approval in May.
Afstyla was originally developed by SK Chemicals and licensed out to Australian biotech firm CSL Ltd. in 2009. Under the licensing deal, CSL has been preparing for the US launch of Abstyla, a longer-acting treatment for hemophilia A which stands superior to existing competitors in the market.
“CSL expects Afstyla will become a strong competitor in the global market for Type A hemophilia treatments,” SK Chemicals said. “In turn, we expect to gain royalties from Afstyla’s global sales.”
Green Cross is also expected to win the US FDA’s approval for its immunodeficiency drug IVIG-SN within next year.
Exclusively developed by Green Cross, IVIG-SN -- human normal immunoglobulin G for intravenous administration -- treats primary immunodeficiency disorders which weaken or rid the immune system’s ability to fight infectious diseases.
The Korean drugmaker was asked to submit supplementary data regarding its biologics license application for IVIG-SN. It expects the drug to be approved without issue once the required additions are filed.
Daewoong Pharmaceutical has also completed phase 3 clinical trials of its botulinum toxin Nabota in the US. It plans to seek the US FDA’s approval of its botulinum toxin production plant within next year, with aims to commercialize the drug in the US in 2018.
By Sohn Ji-young/The Korea Herald (jys@heraldcorp.com)


![[Exclusive] Korean military set to ban iPhones over 'security' concerns](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050599_0.jpg)




![[Herald Interview] Bridging Korea, Philippines for better future](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050735_0.jpg)
