Bio
CSL’s hemophilia drug approved for sale in Europe
[THE INVESTOR] South Korean biopharmaceuticals company SK Chemicals said on Jan. 10 that a hemophilia treatment it licensed out to its partner CSL Ltd. has been approved for sale in Europe, marking another major market entry breakthrough.
The European Medicines Agency, Europe’s drug regulator, granted marketing and sales approval for Afstyla, a biologic drug for hemophilia A, to CSL Behring, a subsidiary of CSL Ltd.
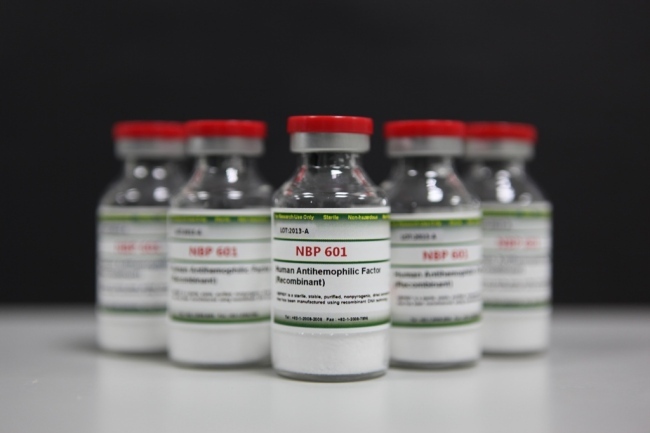 |
Afstyla, or NBO601. SK Chemicals |
Afstyla was originated by SK Chemicals and licensed out to Australian biotech firm CSL Ltd. in 2009. Under the licensing deal, CSL has been carrying out Afstyla’s overseas clinical trials and subsequent approval procedures.
Europe is the third market in the world where Abstyla has been approved for sales. The drug was approved for sale in the US in May 2016 and in Canada in December 2016. The drug is undergoing independent reviews by drug regulators in Switzerland and Australia as well.
Afstyla, also known as NBP601, treats hemophilia A, a congenital blood disorder found mostly among males. Patients with hemophilia A experience prolonged or spontaneous bleeding, as their blood does not clot normally due to a missing or defective factor VIII, an essential blood-clotting protein.
It is the first and only “single-chain therapy” for hemophilia A that acts as a longer-acting substitute for the factor VIII protein. Patients can take Afstyla just twice a week, compared to the three to four injections required by existing drugs.
CSL’s new drug is set to present a new treatment option for European patients with hemophilia A.
According to market research firm Datamonitor, the market for hemophilia A in the top five European Union member states -- Germany, the UK, France, Spain and Italy -- stood at $3.55 billion in 2015.
Meanwhile, the global hemophilia market is expected to reach $15.2 billion by 2024, according to an August 2016 report by Grand View Research.
By Sohn Ji-young/The Korea Herald (jys@heraldcorp.com)


![[From the Scene] KG Mobility poised to take next leap](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/24/20240424050621_0.jpg)





