Bio
Korea approves 1st surgical robot
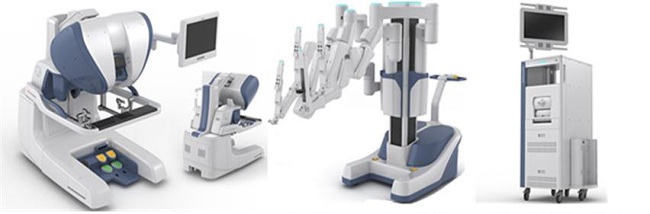
[THE INVESTOR] Korea’s Ministry of Food and Drug Safety said on Aug. 3 it has approved the country’s first surgical robot Revo-i, which facilitates complex surgery using a minimally invasive approach.
Revo-i, developed by Korean biotech firm Meere Company, is designed for use in operation rooms in endoscopic surgical procedures including cholecystectomy and prostatectomy.
 |
“We expect the successful development of the homegrown surgical robot will lead to lower medical expenses and shorter operative time and less blood loss for patients who need such procedures,” an MFDS official said.
During a surgical procedure, a surgeon sits at a console while watching three-dimensional view of patient’s anatomy. The robot will assist in finding the exact area of body for the surgery and also help in making an incision and suturing with its four arms.
Revo-i is the second surgical robot cleared by any health regulator in the world for the use of general minimally invasive surgery, following the da Vinci Surgical System made by the US company Intuitive Surgical and approved by the US Food and Drug Administration in 2000.
So far, the majority of medical robots has been playing limited roles at hospitals such as cutting bones for hip and knee replacement procedures.
According to the MFDS, the global surgical robots market is expected to reach 9.64 trillion won (US$8.54 billion) by 2021 at a compound annual growth rate of 12.1 percent.
Korea’s robotic medical device import increased 34 percent to 19.6 billion won last year from 14.6 billion won in 2015.
By Park Han-na (hnpark@heraldcorp.com)






![[KH Explains] Korean shipbuilding stocks rally: Real growth or bubble?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/25/20240425050656_0.jpg)
![[Hello India] Hyundai Motor vows to boost 'clean mobility' in India](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/25/20240425050672_0.jpg)
