Bio
TissueGene eyes over US$5b annual sales of Invossa
[THE INVESTOR] TissueGene, the US-based biopharmaceutical affiliate of Korea’s Kolon Group, said its cell and gene therapy for degenerative arthritis, has the potential to become a blockbuster drug that could rake in around US$5.4 billion per year.
The Maryland-based company plans to initiate the phase 3 clinical trials of Invossa in the US next year to bring it to the market by 2023.
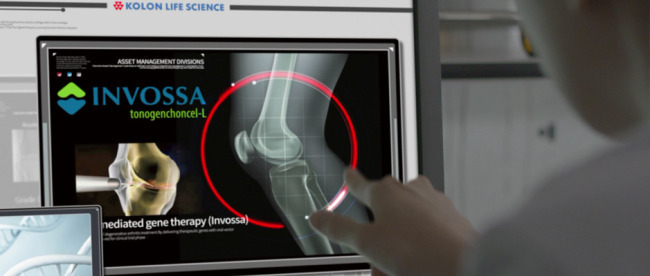 |
The drug is designed as a single injection made directly into the knee joints to induce the regeneration of tissue to treat moderate osteoarthritis. But it has yet to prove that it can help with regeneration, and has so far been approved only as a pain-reducer in Korea.
“We are confident that Invossa will be approved as a disease-modifying osteoarthritis drug by the US health authorities, as it treats the cause of the disease and delays progression,” the company’s CEO Lee Bum-sup said during a press conference on Oct. 16 ahead of its initial public offering next month.
Lee expects Invossa to become the company’s cash cow by 2028. This is based on data showing that the drug, if proved as a radical treatment to prevent the disease from worsening in late-stage clinical studies, can treat up to 11 million patients in the US.
So far, there is no treatment for osteoarthritis. Existing therapies, including steroid injections and other anti-inflammatory drugs, are only used for alleviating pain.
Kolon Life Science, which took 19 years to develop Invossa, is aggressively seeking overseas expansion with the product.
“We plan to begin clinical programs in Japan and China. The European Medicine Agency told us we can receive EU approval based on the clinical data collected in the US,” Lee said.
TissueGene is planning to debut on Korea’s secondary KOSDAQ on Nov. 6, hoping to raise up to 200 billion won (US$176.93 million).
The proceeds from the IPO will be used to fund the planned phase 3 clinical trials in the US, which is expected to cost 100 billion won.
On Oct. 18, the share price of Kolon Life Science, which holds a 31.16 percent stake in TissueGene, hit a three-month high. But industry watchers warn the US-based affiliate will continue to report sluggish financial results for a long time before Invossa becomes a success. The possible failure of last-stage clinical trials also remains a big risk factor.
“The success of the IPO will depend heavily on Invossa’s future value projection,” a source said.
By Park Han-na (hnpark@heraldcorp.com)


![[Exclusive] Korean military set to ban iPhones over 'security' concerns](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050599_0.jpg)




![[Herald Interview] Bridging Korea, Philippines for better future](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050735_0.jpg)
