Bio
Alzheimer’s therapies under development by Korean firms
 |
[THE INVESTOR] A handful of Korean drug makers are scurrying to develop treatments for Alzheimer’s disease as the country faces a rapidly aging population.
On Feb. 5, the Korean government said it will spend 1.1 trillion won (US$1.02 billion) on medical technology to tackle dementia over the next 10 years with some 700,000 suffer from the memory disorder in 2017. By 2026, one in every five South Koreans will be aged 65 or older.
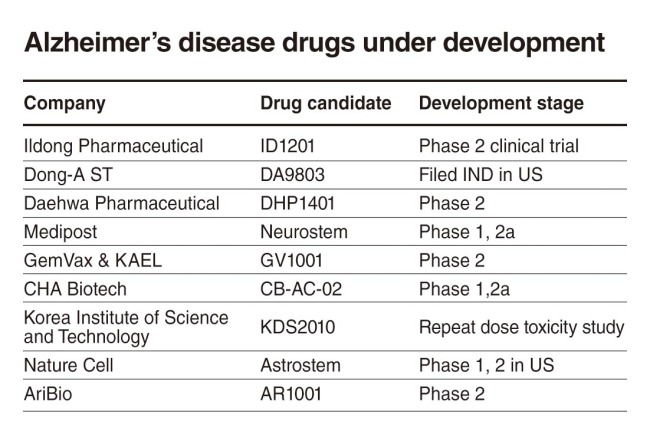 |
Ildong Pharmaceutical
ID1201, the candidate substance extracted from Chinaberry, also known as melia azedarach, for curing Alzheimer’s disease, has been undergoing phase 2 clinical study in Korea since 2014.
The drug candidate has been shown to be effective in preventing dementia by promoting the activity of an enzyme called alpha-secretase, which prevents the formation of beta amyloid -- the main cause of dementia.
Dong-A ST
Dong-A ST has completed preclinical studies on its candidate DA-9803, a therapeutic using herbal extracts and is preparing to file an Investigational New Drug application with the US Food and Drug Administration. In January, the company signed a license deal with NeuroBo Pharmaceuticals to grant the US firm exclusive right to develop and commercialize the herbal drug.
Daehwa Pharmaceutical
The company plans to wrap up phase 2b clinical trials for its DHP1401 comprised of spinosin a C-Glycoside flavonoid extracted from the seeds of Zizyphus jujuba var. In a preclinical mouse model, the therapy was shown to help enhance cognitive performances and adult hippocampal neurogenesis.
Medipost
Medipost’s Neurostem is a mesenchymal stem cell drug derived from the blood of the umbilical cord. On Feb. 5, it received approval from the US FDA to begin early-stage clinical studies of its stem cell-based therapy for Alzheimer’s disease. Preclinical studies have shown that Neurostem is effective in a variety of causative agents and pathological features of dementia such as reduction of amyloid beta protein, inhibition of tau protein hyperphosphorylation and aggregation.
GemVax & KAEL
GemVax & Kael plans to file an Investigational New Drug application for its telomerase peptide GV1001with the US FDA this year. In Korea, the company is recruiting patients with mid- and late-stage Alzheimer’s to conduct phase 2 clinical programs.
By Park Han-na (hnpark@heraldcorp.com)


![[Exclusive] Korean military set to ban iPhones over 'security' concerns](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050599_0.jpg)




![[Herald Interview] Bridging Korea, Philippines for better future](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=151&simg=/content/image/2024/04/23/20240423050735_0.jpg)
