Bio
Korean drugs pending US FDA approval this year
 |
[THE INVESTOR] At least six drug candidates developed by Korean pharmaceutical and biotech companies are looking at possible marketing approval from the US Food and Drug Administration this year.
Making inroads into the US market has been a challenging task for Korean firms due to the high bar and clinical trial costs. So far, only nine products have been given the greenlight since LG Chem’s quinolone antibiotic Factive became the first-ever new drug developed in Korea to receive FDA approval in 2003.
Here’s the list of Korean drugs pending US approval.
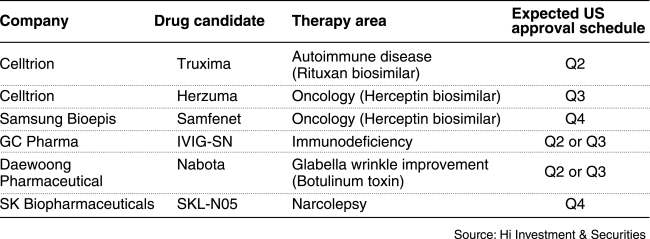 |
Celltrion
This company is seeking to introduce two more biosimilar products in the US following the 2016 launch of Inflectra, its version of J&J’s blockbuster therapy Remicade.
If Truxima and Herzuma clear their final regulatory hurdle, they are expected to put pressure on Roche as the biosimilars are referencing the Swiss drug maker’s Rituxan and Herceptin, respectively.
Samsung Bioepis
Samsung Bioepis’ Samfenet is another knockoff of Roche’s breast cancer treatment Herceptin. The biosimilar will eventually compete with Herzuma, developed by crosstown rival Celltrion in the market. The company has received the European Commission’s marketing authorization for Samfenet in November last year.
GC Pharma
IVIG-SN is one of GC Pharma’s most important pipeline assets, designed to treat primary immunodeficiency disorders that weaken or strip the immune system’s ability to fight infectious diseases. GC Pharma had initially filed IVIG-SN for US approval in 2013 but faced a delay due to manufacturing issues raised during the regulator’s inspection.
Daewoong Pharmaceutical
Nabota is a botulinum toxin Type A developed by the Korean drug maker. While other local peers like Hugel and Medytox are gearing up to make inroads into the world’s largest botulinum toxin market, Nabota is expected to become the first toxin developed by a Korean company to enter the US. If commercialized, California-based biotech firm Alphaeon will be in charge of distributing Nabota in the US.
SK Biopharmaceutical
SKL-N05, an investigational drug to treat narcolepsy, a sleep disorder, is expected to be handed the FDA decision by December this year.
Jazz Pharmaceuticals holds the worldwide development, manufacturing and commercialization rights for narcolepsy drug, with the exception of 12 markets in Asia, where SK Biopharm retains its rights.
The Ireland-based biopharmaceutical company hopes to begin selling the drug in the US from early 2019.
By Park Han-na (hnpark@heraldcorp.com)








