Bio
Samsung Bioepis goes to courts to defeat AbbVie patents covering Humira
[THE INVESTOR] Samsung Bioepis has been mounting a legal challenge to patents held by US drug maker AbbVie on Humira, the world’s best-selling drug, according to an annual report filed by its affiliate Samsung BioLogics on April 3.
Among six ongoing lawsuits involving Samsung Bioepis, five were related to its biosimilar Imraldi, which references AbbVie’s blockbuster immunology med Humira.
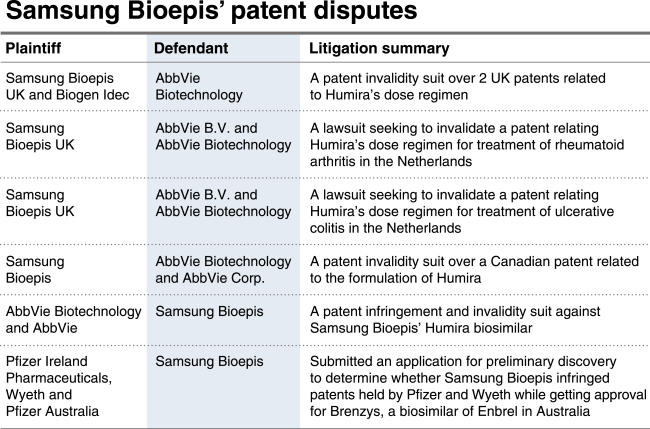 |
Clearing the legal pathway is crucial for the Korea biopharmaceutical company as its version is preparing to penetrate the European market following approval by the European Commission in August last year.
The composition patent for Humira expires in October 2018 but AbbVie has submitted secondary patents in attempts to delay the entry of such biosimilars and protect its own product’s developments such as improved methods of manufacturing, formulations and dosing regimens.
“The launch of Imraldi will come after the main patent covering Humira ends in October,” a Samsung Bioepis official told The Investor.
Samsung Bioepis is targeting the European market, where many of the patent litigations are in play, to chip away at big pharma companies’ dominance in biologic drugs with its set of EU approved biosimilar products -- copycats of Enbrel, Remicade, Humira, Lantus and Herceptin.
AbbVie has also sued the Korean company for patent infringement to protect its top-selling drug as it did for other biosimilar makers including Amgen. In September last year, AbbVie resolved all intellectual property-related litigations with Amgen to delay its US biosimilar until 2023.
By Park Han-na (hnpark@heraldcorp.com)








